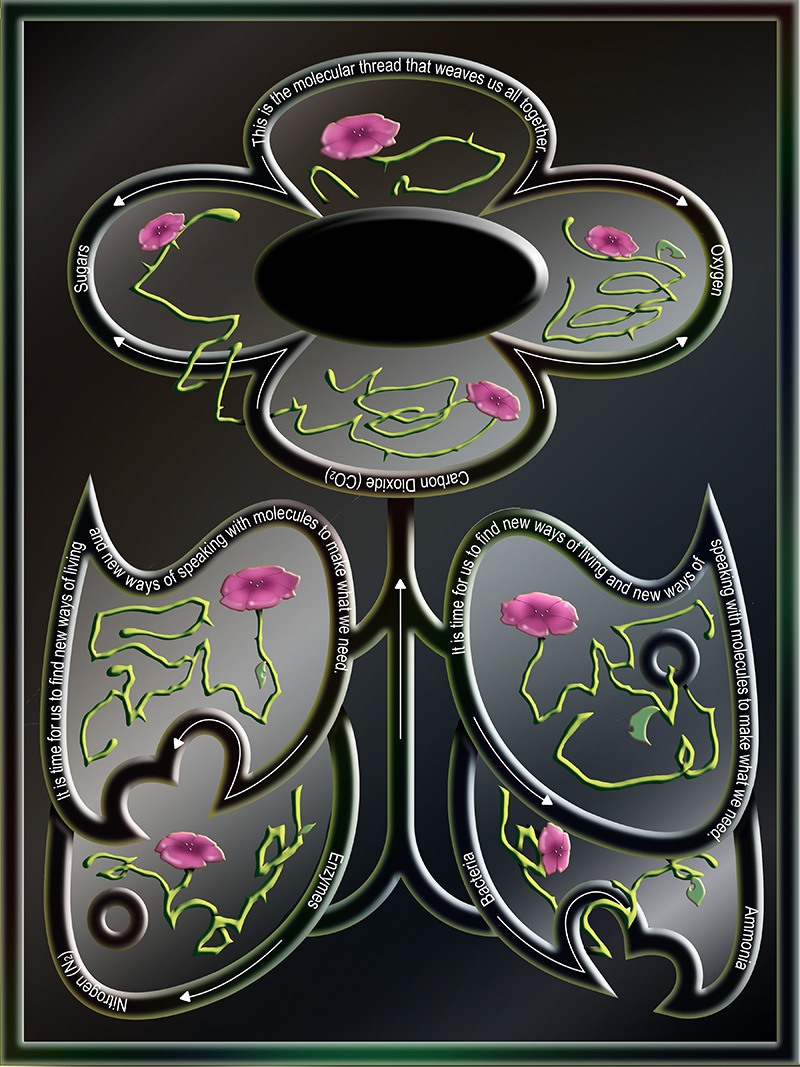
Microbes are Earth’s life support system. They breathe life into the inorganic molecules of our atmosphere, transforming carbon dioxide and nitrogen gas into the backbone of proteins that make up living things.
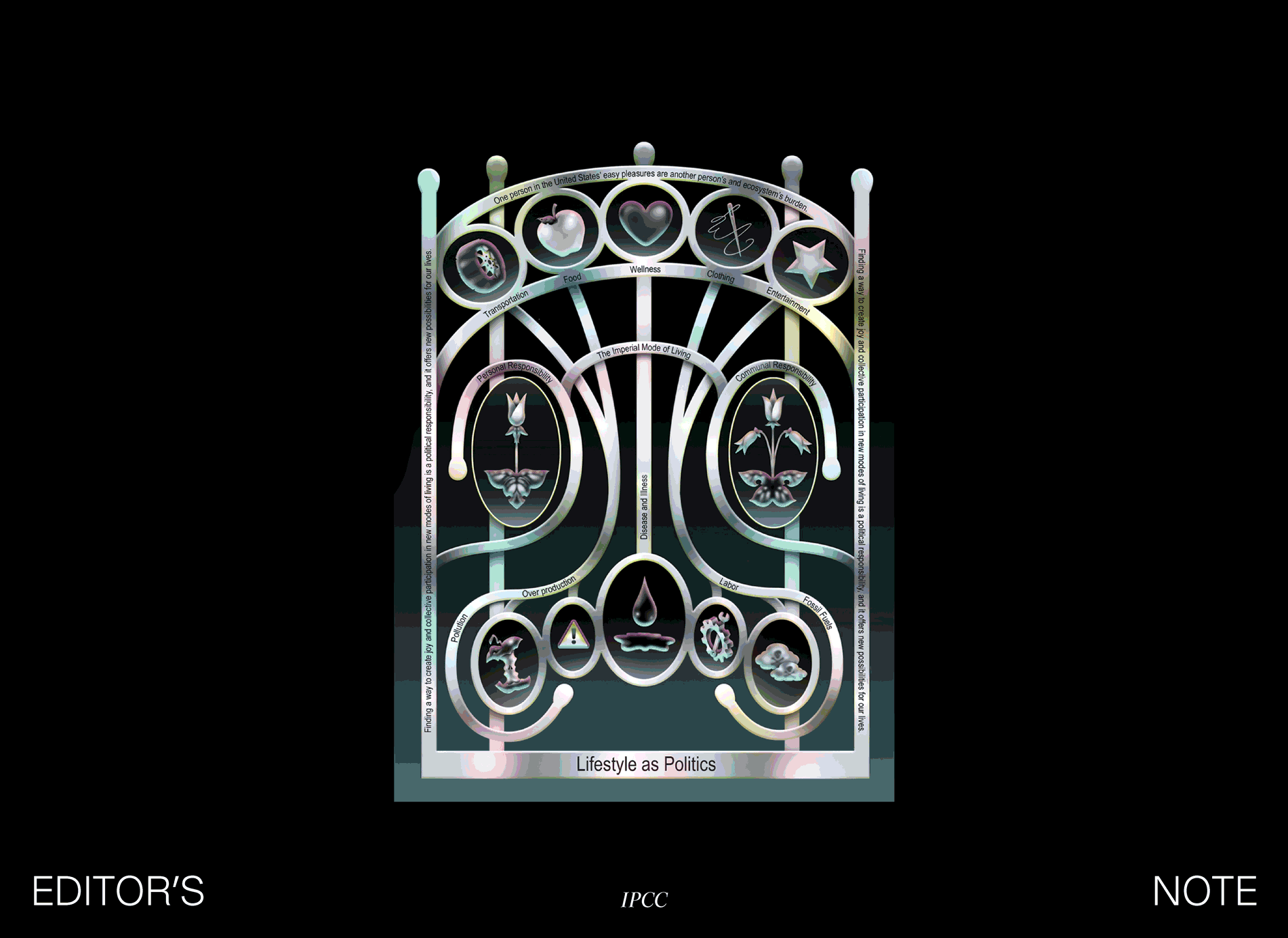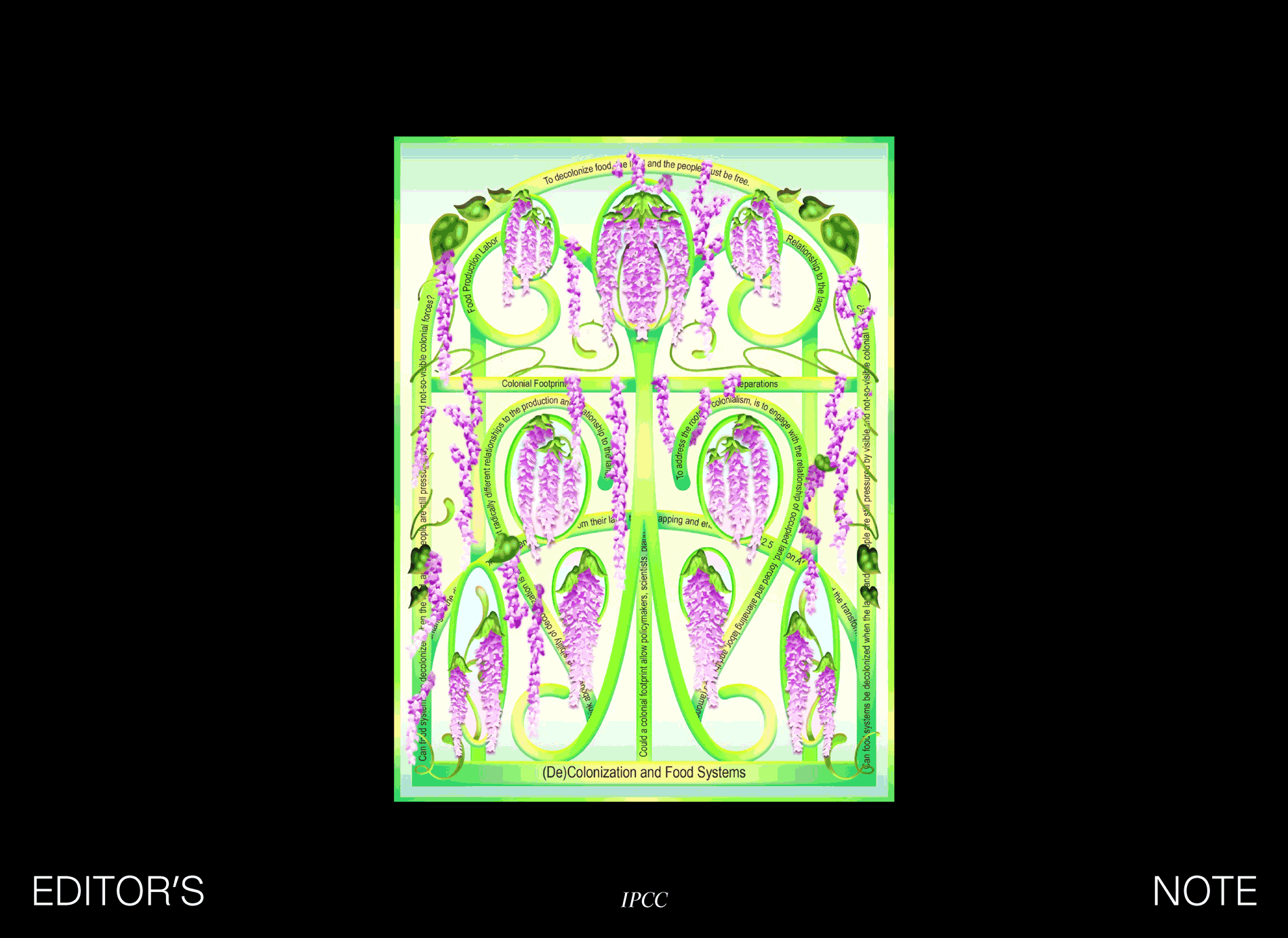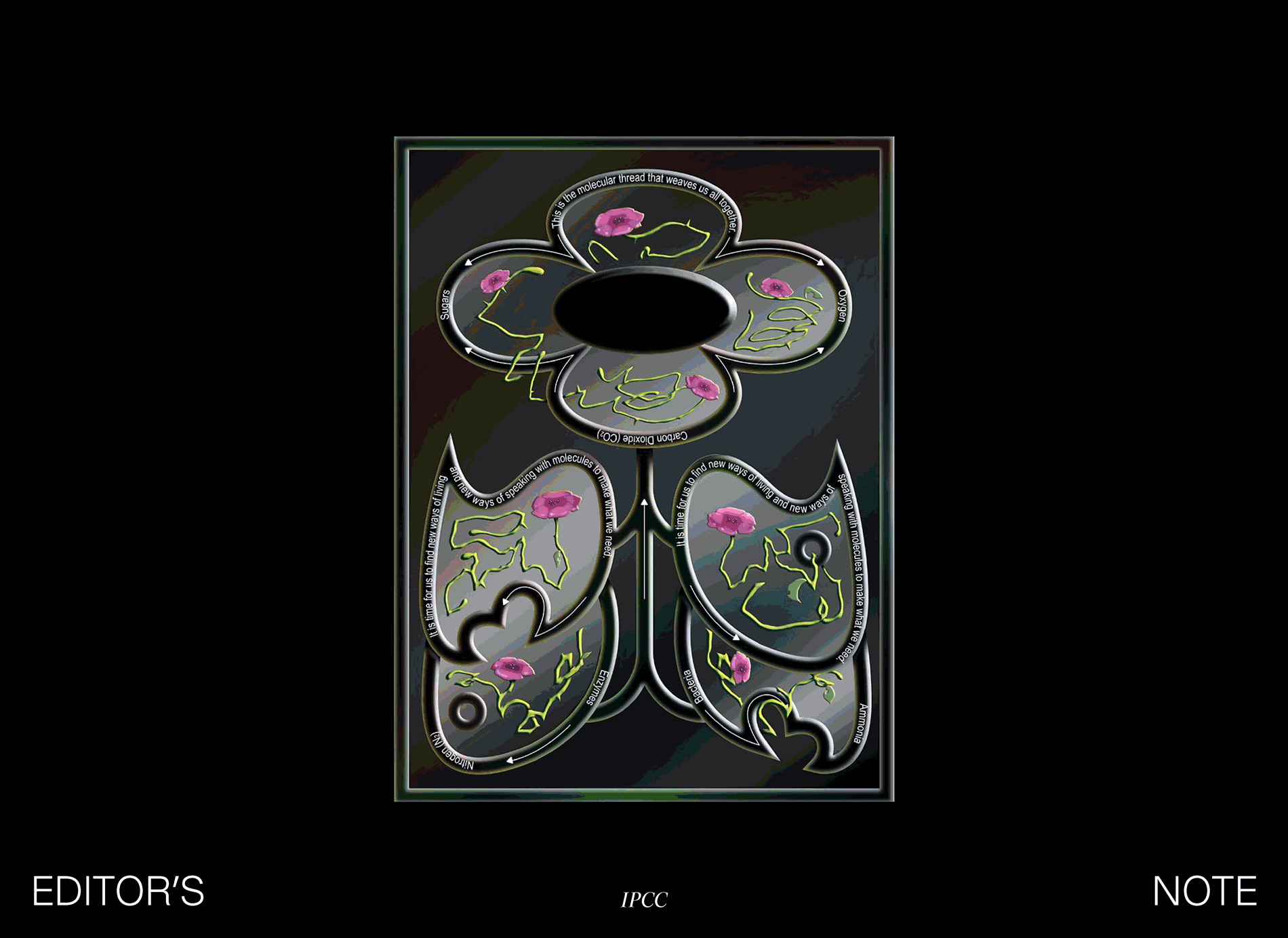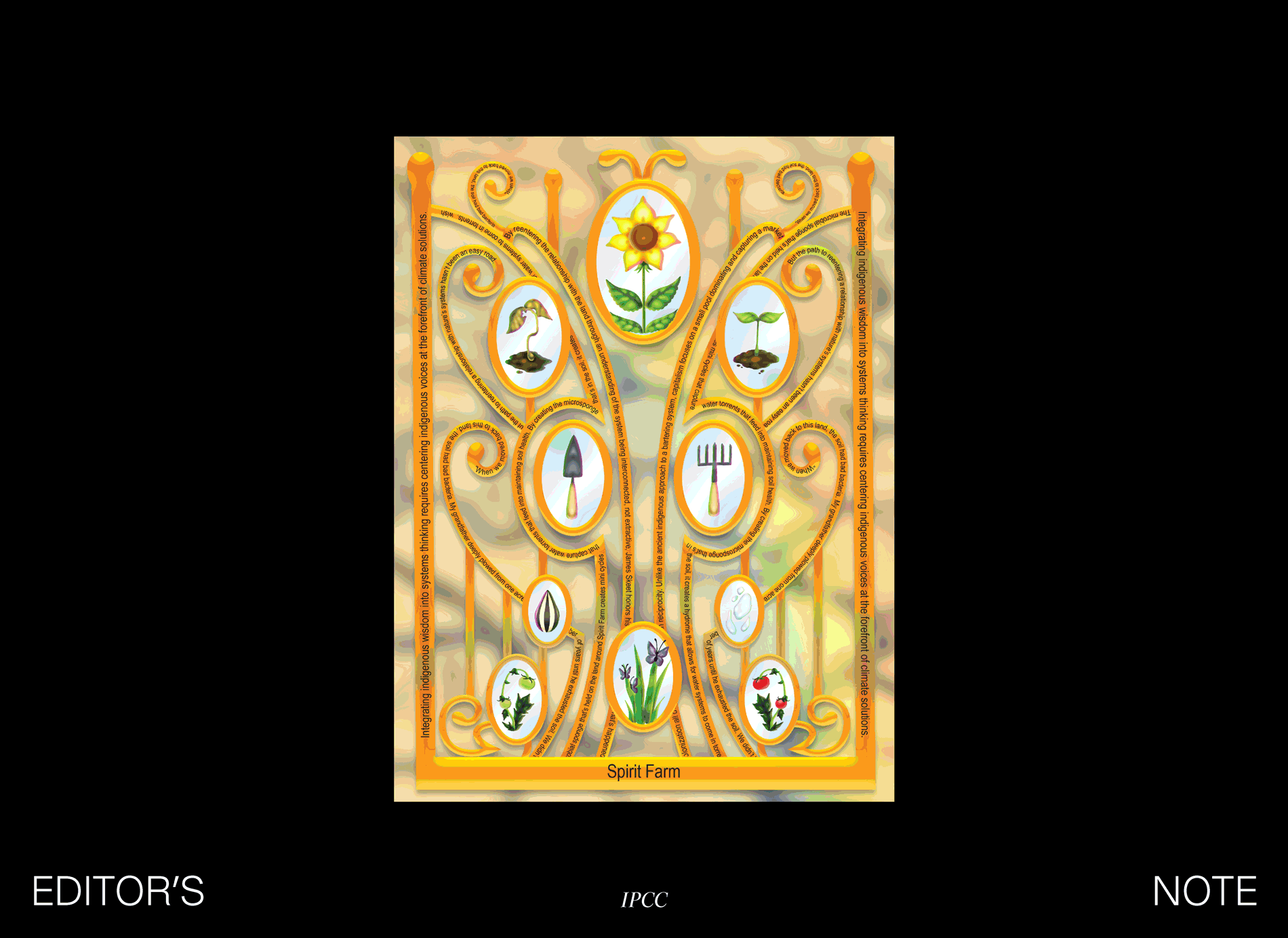
This support system is defined by a series of symbiotic relationships, a networked web of reliance. Carbon flows from the atmosphere through the photosynthetic microbes, algae, and plants that transform carbon dioxide into the oxygen and sugars that make all other organisms’ life possible. At the other end of life, fungi decompose biological matter back into constituent inorganic molecules. This is the molecular thread that weaves us all together.
Nitrogen flows through another set of symbiotic exchanges. Nitrogen makes up the majority of our atmosphere via the inert twinned bond of N2, but the vast majority of organisms can’t use nitrogen in this form. We get our nitrogen from eating plants and animals that have nitrogen already incorporated into their proteins. Plants can absorb nitrogen in the form of ammonia—nitrogen plus hydrogen. Without enough nitrogen fertilizer, plants are yellowed, stunted, unable to thrive and fruit.
Only a few species of bacteria can make ammonia from nitrogen in the air, with specialized nitrogen fixation enzymes that can break and reform the bonds between nitrogen atoms. These enzymes themselves are incredibly complex—to properly build nitrogenase enzymes a whole host of other enzymes are required in order to assemble the various bits and pieces responsible for this incredible chemical reaction. In terms of energy, this complexity makes nitrogen assimilation costly to the organism. The enzymes are also exquisitely sensitive to oxygen—nitrogen fixation must be isolated from the air.
These tools are helping to transform how we produce nitrogen fertilizer, from inside giant chemical plants to directly on the roots of crop plants.
Species of plants from the legume family have evolved a beautiful symbiosis to solve both their needs. The plants need nitrogen in the form of ammonia that bacteria can provide. The bacteria need a safe space where they can perform these reactions away from oxygen and with other needs met. This can happen inside the plant’s roots, in specialized protuberances called nodules, where oxygen is kept out and usable nitrogen can be produced and handed to the plant while the bacteria can be protected from other microbes in the soil and fed sugars through the roots.
Humans have always known about the special magic of plants that don’t need added fertilizer, long before the bacteria responsible were discovered inside the root nodules in 1901. Patterns of symbiotic relationships have been cultivated with our domesticated plants that allow for these biological systems to nourish plants that can’t form symbiotic relationships directly, including the Three Sisters planting method of North American indigenous peoples, crop rotation of alfalfa and soybeans with corn or wheat, or the addition of the fern azolla harboring a nitrogen fixing algae to rice paddies, providing nitrogen to rice plants that don’t form the symbiosis.
Our patterns of symbiosis have radically changed, breaking open the global cycles of carbon and nitrogen. Today we rely on carbon-carbon bonds formed by photosynthesis long ago in the form of fossil fuels. We use these ancient carbon bonds as a blunt tool of human chemistry, and in doing so we unravel the threads of global metabolism. Half of all the nitrogen we need still comes from symbiotic bacteria, but the other half comes from ammonia produced by smashing nitrogen from the air with methane “natural” gas in an industrial process that exhales enormous amounts of carbon dioxide.
Taken together, from the burning of the methane and the release of nitric oxide, fertilizer accounted for 1 billion tons of carbon dioxide equivalent emissions in 2019, or about 2% of global emissions for that year
With industrialization and fossil energy, the global flows of molecules become part of the geopolitical flows of power. Artificial nitrogen fixation was invented first not to feed the world but to enable the production of nitrate gunpowder for the German army in World War I. Before the process was developed and applied to agriculture, tropical islands were colonized and mined for their deposits of guano, rich in nitrogen, potassium, and phosphorus, all necessary for plant growth. The insatiable thirst for fertilizer even led the British empire to grind up Egyptian mummies for the phosphorous present in the bones. Today, the Russian invasion of Ukraine is threatening global food security: as prices on natural gas go up, so too does the cost of nitrogen fertilizer.
Applying this fertilizer to fields is also a blunt tool. Historically, half of fertilizer applied to soils doesn’t get to plants, instead washing out of fields and into waterways where it can wreak havoc on local ecosystems, or breaking down into nitrous oxide, a potent greenhouse gas. Taken together, from the burning of the methane and the release of nitric oxide, fertilizer accounted for 1 billion tons of carbon dioxide equivalent emissions in 2019, or about 2% of global emissions for that year.
Despite these challenges, it is not an overstatement to say that the chemistry that enables the production of synthetic fertilizers has been one of the most important technologies of the last century, making the lives of billions of people possible. These tools of chemistry have made it possible to speak the language of atoms, manipulating nitrogen, carbon, oxygen, and hydrogen. It’s these tools that allow us to transform fossilized carbon-carbon bonds into nearly everything else we use.
It is time for us to find new ways of living and new ways of speaking with molecules to address our present needs. Synthetic chemists speak to the molecular world through heat, solvents, acids, pressure. Synthetic biologists speak to it with DNA. Inside cells, enzymes move atoms and reshape molecules. They take simple sugars and other foods an organism eats and they turn it into more organisms, along with an incredible array of different molecules. Synthetic biologists patch and tweak the sets of enzymes inside of a cell by changing its DNA.
With a fluency in the language of DNA, synthetic biologists are learning to program nitrogen fixation in microbes for other important crops, working with agronomists and growers to reshape how we produce and use fertilizers. These interventions design who fixes nitrogen, when, and where. Some nitrogen fixing bacteria don’t live in nodules, but instead colonize the outside of non-nodulating plants. On the roots rather than inside, nitrogen fixing is blocked, so that the cells don’t waste energy by producing the complex nitrogenases. The generic mechanisms that control that production can be redesigned to activate nitrogen fixing activity on the roots, reducing the need for added fertilizer. The entire nitrogen fixation pathway can also be moved from one cell to another, transferring the ability to fix nitrogen to bacteria that are better suited to life on the roots of crops like corn and wheat. These tools are helping to transform how we produce nitrogen fertilizer, from inside giant chemical plants to directly on the roots of crop plants.
I work for a company whose mission is to make biology easier to engineer—to develop a fluency for the language of DNA and enable new ways of working with biology. We are working with Bayer to integrate all the tools required to understand, design, and deploy agricultural microbes for nitrogen fixation and other key processes in the soil that shape the global metabolism. Over the past four years, our joint venture Joyn Bio has worked to design and test strains of bacteria that can colonize non-legume crops and provide nitrogen, reducing the need for added fertilizer by 40%.
Symbiotic exchanges of carbon and nitrogen make all life possible. Our exploitation of fossilized carbon makes our contemporary life possible, at great cost to the environment that sustains us. It is time for us to now redesign our industrial landscape and renew the ways we live symbiotically with microbes, adapted for a new way of life.