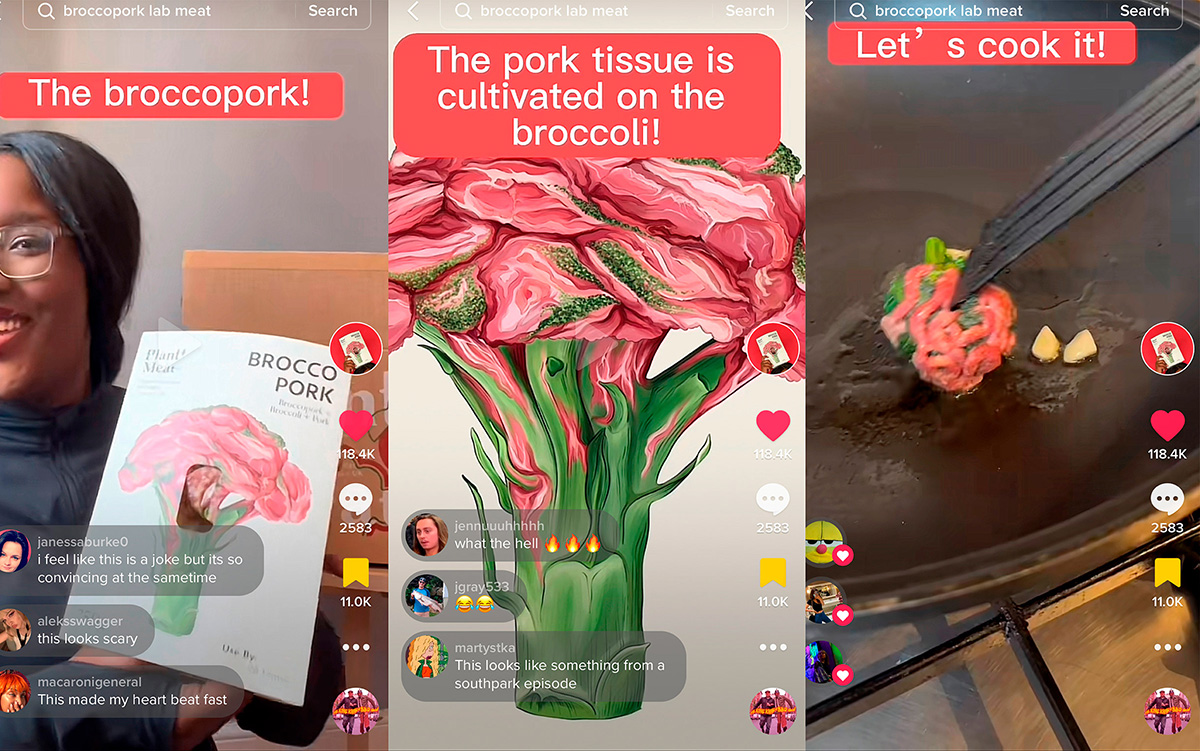
“Protein from petroleum may help solve the world’s food problem,” declared Scientific American magazine in its October 1965 issue.1 The piece, written by lead research scientist Alfred Champagnat, explained that for the first time, scientists were experimenting with microorganisms that can thrive on hydrocarbons and synthesize proteins rich in amino acids that even plant foods lack—a remarkable scientific advancement.
- 1. Champagnat, Alfred. “Protein from Petroleum.” Scientific American, vol. 213, no. 4, Scientific American, a division of Nature America, Inc., 1965, pp. 13–17, http://www.jstor.org/stable/24931150.
In the mid-1960s, the news was met with little interest. In the United States, vegetarianism was still a fringe hippie movement. There was little concern around conventional meat production and its contribution to an unprecedented collapse in the planet’s ecosystems. At the time, the world was burdened by the tensions of the Cold War and the May 1968 youth movements that brought to consciousness the idea of sustainability thinking. Facing a growing world population, the food industry had a determinant role in helping feed the more than 3 billion people who already inhabited the planet.
The fear of an impending food shortage was real, and R&D teams at big companies started thinking about using oil as a food source. Fed with yeast, petroleum creates “single-cell-proteins” that could be used as food. An idea ahead of its time, making it possible to feed thousands of people in a quick, easy, and — hopefully — safe way. Champagnat and his team at British Petroleum (BP) worked on the idea of feeding straight-chain hydrocarbons from their fractionation processes to yeast: they called this the “proteins-from-oil-process.” The company even built the first small-scale pilot plant in 1963 — a dozen years before the process won recognition with the UNESCO Science Prize in 1976.
“Rather surprisingly, this project quickly became the only project of the company’s research organization for quite a few years. This would be unheard of today,” tells Helmut Traitler, co-author of the book Food Industry R&D – A New Approach, in which he describes the background of the development of a technology that never changed the world, as expected. Even if the idea of creating food from petroleum seems a bit absurd, it was very genuine and avant-garde at the time. The urgency of providing sustainable protein alternatives was pressing and the petroleum process uses a lot less water than the equivalent weight in vegetable-based protein, not to mention the 2,000 gallons required to produce just 1 pound of beef. The project for single-cell proteins ran over many years until it was left aside because of other food industry priorities.
More than 60 years have passed and we continue to face the challenge of food security to feed even more people. Over the coming decades, we’ll need to produce twice as much food with much less land and resources. It’s a complex equation to come up with if we consider the social, economic, and primarily environmental costs of producing our food. In regards to meat, the way we produce hasn’t changed much over the last hundred years. Yet the allure of lab-grown alternatives is receiving renewed interest thanks to tech investors and determined scientists.2
- 2. For more on the design possibilities read Benjamin Aldes Wurgaft’s “Designing a New Future for Flesh: The Case for the Carnal Skeuomorph,” from MOLD 04.
In 2013, Mark Post, a tissue engineer conducting research at Maastricht University, presented a radicle burger to a select group of journalists in London. Made using an in-vitro technique to create 20,000 strips of thin muscle tissue to make the five-ounce burger, the cultured beef was grown from the stem cell of a cow costing more than $325,000 in development.3
- 3. https://www.nytimes.com/2013/08/06/science/a-lab-grown-burger-gets-a-taste-test.html
The knowledge and technology behind cultured meat has become exponentially more democratic since then. A single cell could, in theory, produce enough meat (at a much lower cost) to feed the global population for a year, according to New Harvest, an organization funding research into cultured meat. And it may taste, “the same as conventional [meat] because it’s made out of the same stuff,” as New Harvest’s founder, Jason Matheny, pointed out to authors Philip Lymbery and Isabel Oakeshott in the book Farmageddon — The True Cost of Cheap Meat. Adding, “we think we can match the same taste and texture by producing cultured meat in a way that’s much safer, much more efficient, and much healthier for the consumer.
In a devastating report issued last month by the Intergovernmental Panel on Climate Change (IPCC), the United Nations’ climate science research group concluded that climate change has already caused irreversible effects to our planet. Humans are dangerously warming the world through activities like burning fossil fuels and clearing lands for agriculture and urban development. Methane levels are rising fast. The gas is being emitted from various sources: from wetlands and melting permafrost, to agriculture and landfills.
According to the Environmental Defense Fund, how we produce meat is significantly related to rising gas levels: cows release methane, which is 84 times more potent than carbon dioxide. Ditching conventional beef production for cultured meat could be a solution to controlling gas emissions, in some cases with 78-96% lower greenhouse gas emissions despite its dependence on energy consuming bioreactors and other physical and technical infrastructure development requirements.4 The race to develop new proteins has generated a new wave of research with surprising results. Even the seemingly ill-conceived efforts to develop a “proteins-from-oil process” is being revisited by a new generation of scientists in an effort to address food insecurity.
- 4. https://www.eea.europa.eu/publications/artificial-meat-and-the-environment/file
A duo composed of University of Illinois Urbana-Champaign bioengineer Ting Lu and Michigan Technological University biologist Stephen Techtmann engineered microbes to convert plastic into edible protein. Initially funded by DARPA in 2020, the research could provide an answer to the massive churn of plastic production by relying on gene-hacked microbes that can break down the molecules of plastic and non-edible plant biomass and convert it into food for human consumption. As in the case of synthesizing proteins from oil, the “plastics to protein” pipeline may not sound appetizing, but its microbial synthetic biology technology can create the kinds of edible protein that we could be soon served up on the plate.
In one shot, the microbes seem willing to solve two of humanity’s biggest challenges: reduce plastic waste and create access to nutritious food quickly, healthily and cheaply. For the scientists and investors in this new process the hope remains: that 60 years from now, plastic proteins will be consumed for human health instead of being another idea that never made it out of the labs to our tables.
- 1. Champagnat, Alfred. “Protein from Petroleum.” Scientific American, vol. 213, no. 4, Scientific American, a division of Nature America, Inc., 1965, pp. 13–17, http://www.jstor.org/stable/24931150.
- 2. For more on the design possibilities read Benjamin Aldes Wurgaft’s “Designing a New Future for Flesh: The Case for the Carnal Skeuomorph,” from MOLD 04.
- 3. https://www.nytimes.com/2013/08/06/science/a-lab-grown-burger-gets-a-taste-test.html
- 4. https://www.eea.europa.eu/publications/artificial-meat-and-the-environment/file